The U.S. Food and Drug Administration (FDA) has seemingly removed any objection to the Pfizer COVID vaccine by approving the company’s biologics license application. That gives the gene-altering serum general-use approval for individuals age 16 and over, as opposed to the more restrictive Emergency Use Authorization (EUA) assigned to other COVID vaccines.
EUA products are experimental, meaning that under U.S. law, everyone has a right to refuse them. Forcing someone to get the experimental jab so they can work or go to school is tantamount to Nazi human experimentation during World War II, and is in direct violation of the Nuremburg Code.
FDA’s approval removed that stigma.
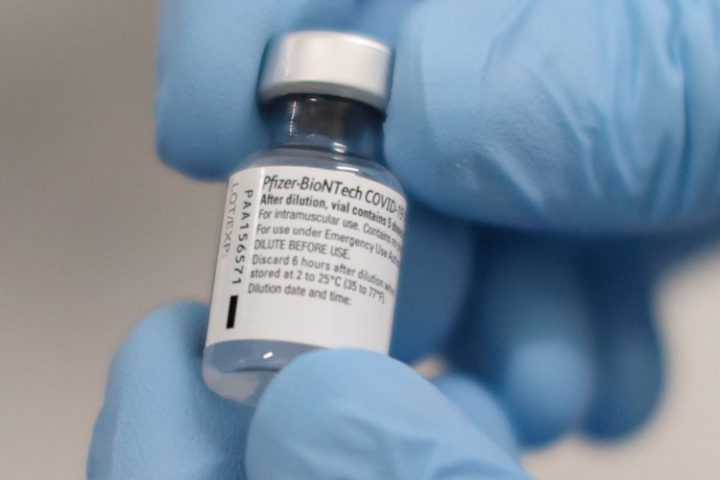
Here’s the catch: The FDA only licensed Pfizer’s Comirnaty vaccine, not its BioNTech product, which remains under EUA restriction. Confusing the matter further, in its approval documentation, FDA acknowledges “insufficient stocks” of the Comirnaty vials, but “sufficient amounts” of BioNTech, claiming the products can be used “interchangeably.” FDA chief scientist Denise Hinton wrote: “The licensed vaccine has the same formulation as the EUA-authorized vaccine and the products can be used interchangeably to provide the vaccination series without presenting any safety or effectiveness concerns. The products are legally distinct with certain differences that do not impact safety or effectiveness.”
Did the FDA just equate an EUA vaccine to one that is fully licensed? Let’s look at the “legally distinct” differences. First, U.S. law permits employers and schools to require licensed vaccines. Second, while manufacturers of EUA-approved vaccines are immune from product liability (as are those who produce vaccines placed on the FDA’s mandatory childhood immunization schedule), licensed adult vaccines do not enjoy liability protection. They are subject to the same product liability as any consumer goods in the U.S. market. In other words, if you were injured by the Comirnaty vaccine, you could sue. Injured by BioNTech? No chance.
So why would Pfizer market Comirnaty in the United States, where it can end up in court when patients are injured? With “significant amounts” of BioNTech available, written FDA go-ahead, media silence on this criminal sleight-of-hand, and general public ignorance of what amounts to illegal mandates resulting from FDA’s approval, Pfizer can unload all its liability-immune product on an unsuspecting public while President Biden, in Hitleresque fashion, orders private companies nationwide to mandate the jab.
What’s particularly disconcerting is that there are more reported deaths attributable to Pfizer vaccines than to the others combined. The Vaccine Adverse Event Reporting System (VAERS), operated jointly by the FDA and the U.S. Centers for Disease Control (CDC), is a voluntary reporting system designed to detect possible early warnings in regard to vaccine safety. VAERS records almost 10,000 deaths associated with Pfizer’s product, whereas deaths reportedly caused by other manufacturer’s vaccines amount to 4,500. Overall VAERS has received more than 675,000 reports of adverse events, including nearly 20,000 that involve permanent disability and tens of thousands of life-threatening events.
Contrast this with past VAERS experience. “A typical new drug … at about 50 unexplained deaths for a new product, it’s pulled off the market,” Dr. Peter McCullough told Alex Newman of The New American. “In the 1976 swine flu pandemic we attempted to vaccinate 55 million Americans. At 25 deaths, the program was killed.” Dr. McCullough is a principal faculty in internal medicine at Texas A&M University Health Sciences Center and developed a successful COVID outpatient treatment early in the pandemic.
Want more undiluted proof of Pfizer’s track record? Let’s look at Israel, where the government cut an early exclusivity deal with Pfizer, paying above-market value for premium access to its vaccine. The Pfizer CEO blatantly called Israel “the world’s lab.”
What are the lab results? Journalist Sharyl Attkisson has kept a running tally of COVID-19 vaccine concerns on her blog, where Israel is heavily featured. For example, a recent case-control study from that country found an increased risk of myocarditis (heart inflammation), appendicitis, lymphadenopathy (inflammation of lymph nodes), and herpes zoster infection (shingles). Israel’s Ministry of Health has admitted that nearly half of adults infected with the COVID-19 virus are fully vaccinated, and that 60 percent of hospitalizations involve fully-vaccinated individuals. A spokesman for Israel’s largest health maintenance organization called their Pfizer experience “a very clear warning sign for the rest of the world.” So Israel has lifted mandates, right? On the contrary, its reaction is unbelievable: The country now only rewards vaccine passports to those who have had three shots, not a mere two, and the passport is only good for six months.
Attorney Robert F. Kennedy, Jr. gives this advice: “Here’s what you need to know when someone orders you to get the vaccine: Ask to see the vial. If it says ‘Comirnaty,’ it’s a licensed product. If it says ‘Pfizer BioNTech,’ it’s an experimental product, and under 21 U.S. Code 360bbb, you have the right to refuse.”
Of course, all other so-called COVID vaccines are still under EUA restrictions, so you have the right to refuse those as well.




