
Editor’s Note: This article is the second in a continuing series published by The New American. For background material, please reference our first article, Dispelling Irrational Fear of Radiation.
We live in a radioactive world where we are all exposed to ionizing radiation every day of our lives. It is called natural background radiation and varies around the world. Sources of background radiation are illustrated in the pie chart below.
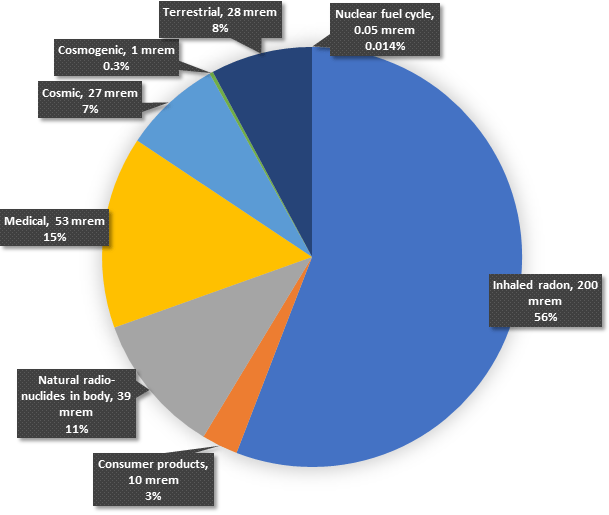
Our Radiation Environment
This chart shows that the average American receives a total equivalent dose of around 360 millirem (mrem) per year. A millirem is a very small unit of measure. To better understand the relative insignificance to human health from a millirem of radiation, consider these examples: The average dose from watching color TV for a year is 2 mrem. Passengers on a round-trip transatlantic flight receive a radiation dose of about 5 mrem just due to the plane’s altitude above the Earth, which exposes them to more cosmic radiation. Employees of New York’s Grand Central Station are exposed to about 120 mrem each year from the uranium and thorium in the granite used in the building’s construction. Radiation exposure for residents of Denver, Colorado, is about 50 mrem more each year than residents of Los Angeles, California, because of Denver’s greater altitude.
Depending on one’s location in the world, the individual contributors to background radiation dose may vary somewhat in percent, and more so in quantity, from the representative numbers shown in the pie chart.
Terrestrial radiation is from radioactive material in the ground beneath us. This material is primarily the product of uranium and thorium radioactive decay. Both of these elements are plentiful in the Earth’s crust, and because they have been decaying since the Earth’s formation, there has been a substantial buildup of the radioactive isotopes produced in their decay chains.
Cosmic radiation comes from the Sun and other radiating objects in the universe, such as supernovas that produce extremely high-energy, heavy, charged particles. Cosmogenic radiation is radiation resulting from cosmic ray interactions with atoms in the Earth’s atmosphere.
Radioactive material contained in the human body is dominated by potassium-40 and carbon-14, radioactive isotopes of potassium and carbon, respectively.
Radiation is received from consumer products such as color television sets, clocks and watches, and various building materials such as cement, concrete, and gypsum, which contain uranium and thorium.
Medical radiation consists primarily of diagnostic x- and gamma-rays.
It is quite evident from the pie chart above that the largest single contributor to total background radiation dose is radon. Radon is a gas produced in the uranium and thorium radioactive decay chains — the primary sources of terrestrial radiation. Because it is a gas, radon migrates up through the soil and becomes part of the air we breathe. Despite claims made by various organizations, radon is not the leading cause of lung cancer in non-smokers.
Some of what we eat is also radioactive, with potassium-40 being the primary contributor because potassium is so pervasive in our environment. Examples of foods containing potassium-40 include Brazil nuts (which also contain radioactive radium-226), lima beans, bananas, white potatoes, carrots, and red meat.
Radiation Risk
We need to remember what Paracelsus once said: “The dose makes the poison.” This is also true of ionizing radiation. While high doses can cause cancer, low doses are not health hazards, as evidenced by chronic exposure to natural background radiation.
As indicated in a previous article, alpha radiation has a very high linear energy transfer (LET) and therefore relatively low penetrating power. In fact, alpha radiation has a range in air of only one and one-half inches. That is, interactions with air molecules causes alpha radiation to lose all of its energy in about one and one-half inches of travel from the source. Alpha radiation can be effectively blocked by cardstock material and cannot penetrate the dead layer of human skin.
Although beta radiation has a much larger range in air and therefore more penetrating power than alpha radiation, it can be blocked by a sturdy sheet of plexiglass. With the exception of very high-energy radiation, beta radiation also cannot penetrate the dead layer of human skin.
Both gamma-rays and x-rays can penetrate the human body, which is why they are used to image internal body organs.
So, although external exposures to alpha and beta radiation are not hazardous to health, exposure to gamma-rays and x-rays may present a health hazard, depending on the energy and the exposure time.
Read the previous article in this series, Dispelling Irrational Fear of Radiation, and the subsequent article, The Dynamic World of Radioactive Decay.
Jeffrey Mahn is a retired nuclear engineer, and currently volunteers as a docent and educator at the National Museum of Nuclear Science and History in Albuquerque, New Mexico.





