
In a newly published editorial in The New England Journal of Medicine, FDA commissioner Dr. Marty Makary and his chief regulatory official, Dr. Vinay Prasad, outlined what they call a “new,” “evidence-based” Covid vaccine philosophy.
The agency proposes a three-tiered regulatory model for future booster approvals.
Under this framework, vaccines for adults over 65 and those labeled “high-risk” will continue to be authorized based solely on immunogenicity — the ability to trigger an antibody response, without evidence of clinical benefit. For healthy individuals, future approvals will require data from randomized, controlled trials.
But the appearance of scientific rigor masks a more troubling reality. The FDA is not scaling back the program. They preserve it — and redirect it with surgical precision toward the most vulnerable: the chronically ill, elderly, sick infants, and pregnant women.
As Makary and Prasad concede, “The American people, along with many health care providers, remain unconvinced” they need another dose. In that light, the FDA’s updated strategy appears less about reform and more about justifying the program’s continuation under the banner of evidence generation.
The Strategy
The new framework puts the population into three buckets. Here is how the FDA claims it will proceed:
“High-risk” Groups
The FDA now defines “high-risk” — meaning eligible for another booster — so broadly it includes from “100 to 200 million Americans.” According to Makary and Prasad, people aged 6 months to 65 years qualify for boosters if they have at least one condition linked to severe Covid outcomes. The list includes cancer, heart and lung disease, HIV, diabetes, obesity, depression, mood disorders, Down syndrome, and current or recent pregnancy, among others.
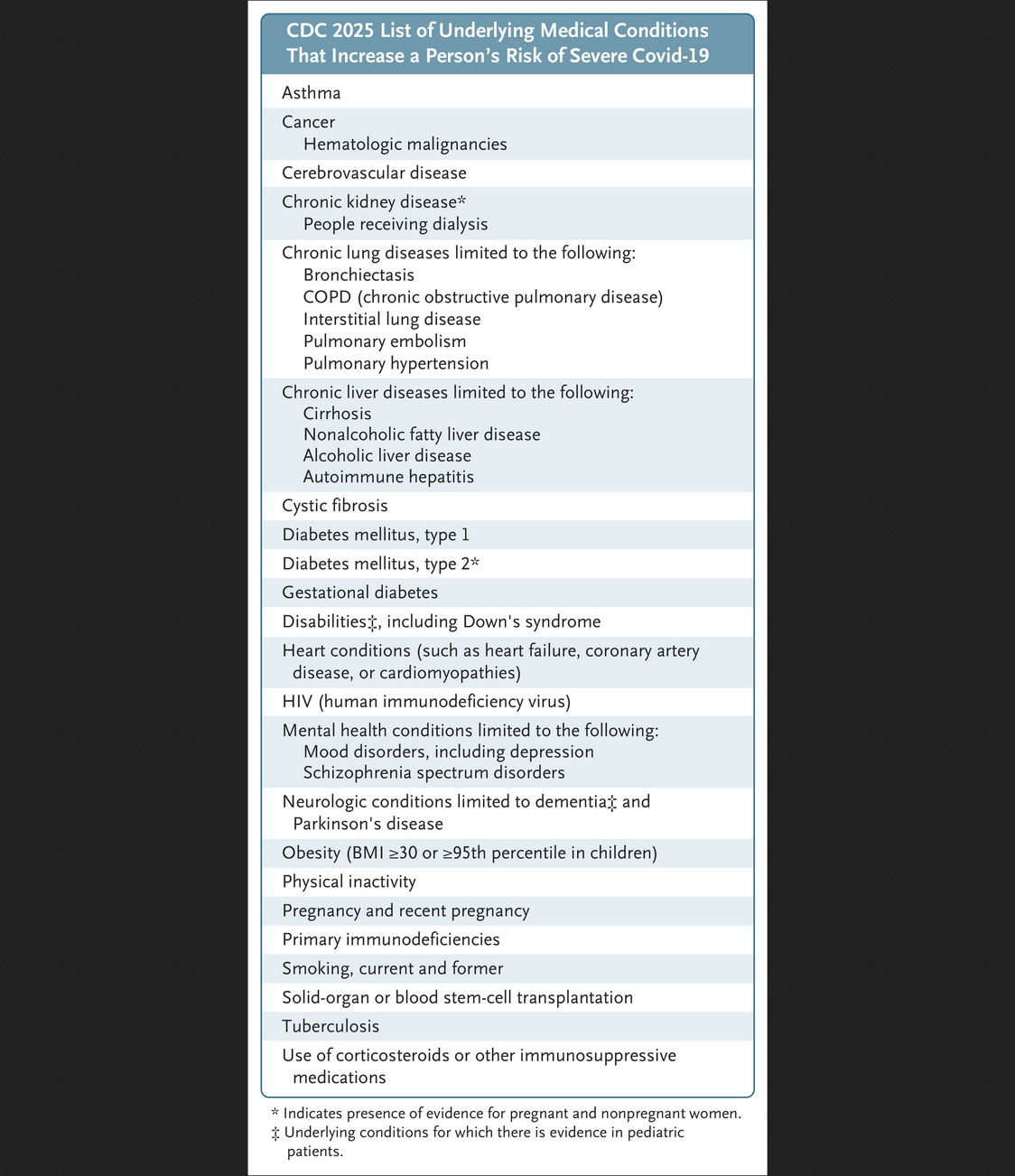
In addition, all adults over 65 are eligible by default.
For these groups, Covid boosters will be authorized just as before, solely on immunogenicity. Immunogenicity refers to a vaccine’s ability to trigger an immune response, usually measured by the presence of antibodies in the blood. But antibodies alone don’t provide protection. A high antibody count doesn’t guarantee that a person won’t get infected, get seriously ill, or die from the disease. That’s because the immune system is complex, and not all antibodies are protective.
Put simply, a vaccine can look good in a lab by boosting antibody levels, but still fail to prevent illness in the real world. This is why randomized, controlled clinical trials — which measure actual outcomes like hospitalization and death — are essential. Without them, approving a shot based only on antibody production ignores the real question: Does it help more than it harms?
No randomized trials will be required in the “high-risk” population itself, and the FDA will merely “encourage” manufacturers to run postmarketing trials in healthy adults after the shots have already been rolled out to the vulnerable.
Makary and Prasad offer no evidence that additional Covid boosters benefit “high-risk” groups.
Moderate-risk Group: FDA’s Testing Ground
Americans aged 50 to 64 without underlying conditions now occupy a strange regulatory limbo. The FDA calls them an “ideal group” for future clinical trials — not because boosters have shown benefits, but because global health authorities remain divided on whether this group needs them at all. Makary and Prasad write:
Given that there is global equipoise about yearly boosters for the 50-64-year-old age group, we assert that this is an ideal population for future trials.
The FDA says these studies should focus on symptomatic Covid as the primary endpoint, while tracking hospitalization, severe illness, and death as secondary measures. Trials should last at least six months, include previously infected individuals, and use saline placebos to fully document adverse events.
These aren’t minor tweaks. They’re a crucial admission: No robust trials have ever supported boosters in this age group, and the current policy has never been grounded in long-term safety or efficacy.
Nonetheless, the FDA doesn’t recommend pausing booster rollout. The injections will continue; the science is expected to catch up later.
Healthy Americans (6 months to 64 years)
For this final group, the FDA says it will now require actual clinical-trial evidence before granting a full licensure. On the surface, that sounds reasonable. But in practice, it paves the way for unethical trials on healthy individuals, including children, to justify a product already proven unnecessary and harmful.
Rebranding a Disaster Doesn’t Fix It
Corporate media framed the FDA’s policy shift as a “restrictive” step. Yet the medical-freedom movement saw it for what it is: a rebrand, not a rollback.
Dr. Nicolas Hulscher of the McCullough Foundation condemned the strategy as one that “disregards millions of American deaths, injuries, and disabilities.” In a widely shared Substack post, he cited two independent mortality models — one based on adjusted data from VAERS (the Vaccine Adverse Event Reporting System), the other from the Massachusetts Institute of Technology and the Florida Department of Health — estimating between 470,000 and 600,000 American deaths from Covid vaccination.
Hulscher wrote:
When a genetic product causes more American casualties than World War I, World War II, and the Vietnam War combined, basic common sense dictates that its use must be halted entirely — not continuously administered to “high-risk” individuals.
The damage goes far beyond mortality. Hulscher’s sources highlight:
- Over 37,000 deaths logged in VAERS — exceeding historic recall thresholds by 375,000 percent
- Severe adverse events in immunocompromised patients, children, and the elderly
- DNA contamination found in vaccine vials across multiple countries and platforms
- Negative efficacy — studies show higher infection risk among the recently boosted
- Excess mortality — more than a dozen studies suggest up to 17 million deaths globally from Covid shots
His conclusion is unequivocal:
The COVID-19 shots must be removed from the market. Not rebranded. Not reauthorized. Removed.
A Philosophy of Evasion, Not Evidence
In the closing of their piece, Makary and Prasad attempt to distinguish Covid from influenza to justify a separate regulatory track. They admit SARS-CoV-2 mutates differently, has robust summer transmission, and may not require annual vaccine updates. They even concede that natural immunity “appears” to offer “robust” protection against severe illness. That is a fact long established and long ignored.
But instead of following this logic to its conclusion — that mass boosting may be unnecessary, ineffective, or even harmful — they pivot to defending the FDA’s “new philosophy” as a balance between “regulatory flexibility” and scientific rigor.
In practice, it’s neither. The FDA promises “gold-standard” data for healthy Americans, while continuing to greenlight injections for the sick, pregnant, elderly, and infants without clinical trials.
The article ends with a tired refrain — that future studies will bring clarity “desperately craved” by healthcare providers and the public.
Yet, a health-first FDA would halt the campaign until it has solid proof of safety and efficacy. Instead, it keeps the program running and hoping no one notices the evidence is still missing. An agency never envisioned by the Constitution once again appears to enable corporate interests and promote what many doctors have called crimes against humanity.
Still Shielded From Liability
It is important to note that all Covid vaccines and boosters remain fully shielded from liability. Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. — who once condemned the vaccination campaign as “mass murder” — now presides over its continuation. Rather than dismantling the program, his agency is sustaining it.
Kennedy holds the authority to amend or revoke the Public Readiness and Emergency Preparedness (PREP) Act, the legal shield protecting manufacturers through December 31, 2029. He has done nothing.
At the same time, the FDA continues to authorize key products — including booster and pediatric Covid shots — under Emergency Use Authorization (EUA). Under Section 564 of the Federal Food, Drug, and Cosmetic Act, Kennedy could revoke those EUAs today. Yet he doesn’t.
Instead, his HHS is actively expanding the program through its $4.7 billion “Project NextGen.” That is a next-generation Covid vaccine initiative backed not only by taxpayer dollars, but, reportedly, by funding from Bill Gates and the Rockefeller Foundation.
Related:
FDA Deploying AI to Accelerate Drug Approvals
Trump Nominates Biotech Insider Susan Monarez to Lead CDC
HHS Continuing mRNA Research Funding
RFK, Jr.’s Covid Optics: One Trial Paused, Vaccine Agenda Advancing
RFK Jr. Calling for MMR Vaccinations. What About His Past Safety Warnings?





