Editor’s note: Radiation can make you sick, give you cancer, and even kill you, so we avoid it at all costs. We don lead aprons for dental x-rays while techs duck behind metal partitions to dodge atomic-induced cancer and the possibility of future mutant children. We picket with NIMBY placards in opposition to nuclear power plants and dread environmental contamination. We cringe at the prospect of CT scans, and at names such as Three Mile Island, Chernobyl, and Fukushima.
This “common knowledge” of radiation risk is based on a decades-old, debunked misconception known as the “linear, no-threshold” model (LNT). It erroneously asserts that even small amounts of radiation are detrimental, and the higher the exposure, the greater the risk of lethal effects.
“While it is unquestionable that very high levels of radiation can cause death, illness and the increased risk of cancer, there is unimpeachable evidence that low levels of radiation are not harmful to human life at all,” writes Ed Hiserodt in his 2005 book Underexposed: What If Radiation Is Actually Good for You? Low radiation levels even contribute to optimal health.
Conversely, fear of radiation can be deadly. The charter of the nonprofit Scientists for Accurate Radiation Information (SARI) states, “Misinformation related to low-level radiation exposure leads to unintended fatalities.” It cites 2012 research published in the Dose-Response journal that “fear based on the LNT was responsible for about 100,000 abortions following the Chernobyl accident and is now also responsible for more than 1,000 lives being lost related to Fukushima evacuations.”
Unfortunately, LNT still holds sway in U.S. nuclear regulatory policy, and mainstream media and scientific journals are awash with misinformation that perpetuates irrational fears.
To help dispel these myths, The New American is beginning a series featuring reliable nuclear news. Our correspondent, retired nuclear engineer Jeffrey Mahn, currently volunteers as a docent and educator at the National Museum of Nuclear Science and History in Albuquerque, New Mexico.
There are many types of radiation, which is a term that refers to the emission of energy in the form of waves or particles. We encounter radiation each day, such as that used in broadcasting (e.g., radiowaves) or in the home (e.g., kitchen microwaves, heat lamps, cell phones). These are forms of non-ionizing radiation, because none of them have sufficient energy to remove electrons from atoms. Below, Mahn explains the terms and metrics of ionizing radiation, which we also encounter each day: in our food, from building materials, and even in our own blood. His tutorial will assist in future articles with understanding not only the true risks of ionizing radiation, but also its spectacular benefits.
The α β γ’s of Radiation
Ionizing radiation has sufficient energy to dislodge orbital electrons when interacting with atoms, thereby creating positively charged ions. Hence the name, ionizing radiation.
The forms of ionizing radiation to which humans are commonly exposed are called alpha, beta, x-ray, and gamma-ray. The first two forms, alpha and beta, are subatomic particles that have mass. Alpha radiation is a helium nucleus (two protons and two neutrons), and beta radiation is an electron. Both originate from radioactive decay of matter.
X-ray and gamma radiation are electromagnetic waves that have no mass. Like alpha and beta radiation, gamma rays originate from the radioactive decay of matter, while x-ray radiation is generated in x-ray tubes.
Measuring the Invisible
We quantify radiation exposure using metrics that characterize the energy deposited from radiation interactions with matter. The metrics associated with such interactions are called linear energy transfer (LET) and the radiation absorbed dose (rad). LET is the energy deposited by ionizing radiation per unit distance of travel through matter, and absorbed dose is the energy deposited in matter per unit mass of material. The biological effects of radiation depend on both its LET and the absorbed dose.
Alpha particles, being massive compared to beta particles, have a very high LET in matter and therefore relatively low penetrating power. (Hiserodt puts this concept in layman’s terms: “In the subatomic world, the emission of an alpha-particle is like shooting a shot put with a sling shot: there is a lot of mass involved, but it doesn’t travel very far.”) Beta particles and gamma-rays have very low LET in matter and therefore much higher penetrating power than alpha particles.
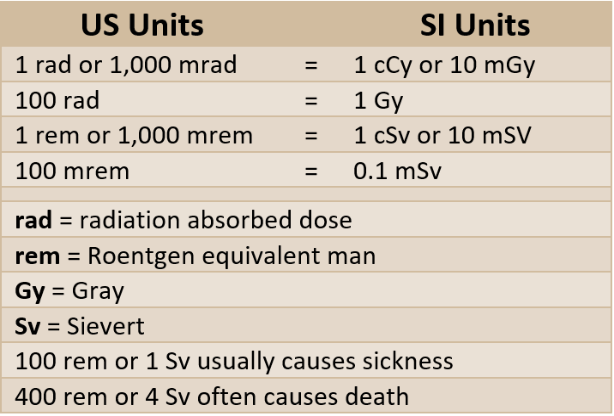
Absorbed doses of radiation are measured in units called rads or Grays. The difference is that the rad is the traditional unit for radiation absorbed dose, and the Gray (Gy) is the International System unit. One Gy is equivalent to 100 rad. While these units apply to any radiations and materials, they are used primarily with biological systems.
Biological Effects
For biological systems, it has been convenient to define a relative biological effectiveness (RBE) for the various types of radiation. This accounts for the different effects that each type of radiation — alpha, beta, x-ray or gamma — exerts on an organism.
RBE also depends on the dose, the dose rate, and other factors. The upper limit of RBEs for a specific type of radiation is called the quality factor (QF). In 1977, the International Commission on Radiological Protection (ICRP) recommended using quality factors of one (1) for x-rays, gamma-rays, and beta particles, and 20 for alpha particles.
We can multiply the quality factor by either rads or Grays to come up with a much more useful metric called equivalent dose. Why is it more useful? The equivalent dose accounts for the biological effect of ionizing radiation, no matter whether its form is alpha, beta, x-ray, or gamma.
If we only referred to radiation in terms of rads or Grays, we would always have to specify the type involved. Instead, equivalent doses account for each quality factor, and can therefore easily be added together to describe the cumulative effect of all radiation exposure. Equivalent doses of radiation are expressed in units called rems or Sieverts.
A rem is the traditional unit of equivalent dose. It is equal to the dose in rad multiplied by the quality factor for the radiation type. For low LET radiation (beta, x-rays and gamma-rays), one rad is equal to one rem.
Of course, there is also an International System equivalent dose: a unit called the Sievert (Sv). The dose in Sievert is equal to the dose in Gray multiplied by the radiation type quality factor. Similar to the traditional system of measurement, for low LET radiation (beta, x-rays and gamma-rays), one Sievert is equal to one Gray.
There is also a very convenient conversion factor between traditional and International System units: 100 rems equal one (1) Sievert.
Latest Research
More recently, the use of quality factors has come under some criticism for not accurately characterizing actual biological effects. Tissue weighting factors have been proposed as being more appropriate to use for calculating dose, so that dose is then dependent on the specific biological tissue receiving the exposure. (Some tissues are more susceptible to radiation damage than others.)
In 2007, the ICRP recommended that equivalent dose in a tissue or organ be weighted to represent the relative contribution of that tissue or organ to the total biological effect resulting from uniform irradiation of the body, where the sum of tissue weighting factors is equal to one.
When It Hurts, When It Helps
A dose accumulated over a very short period of time is said to be an acute dose, while one accumulated over an extended period of time is said to be a chronic dose (e.g., natural background radiation from things such as soil, food, water, and air). Though the word “chronic” makes it seem bad, natural background radiation is not only harmless, but also necessary to maintain optimal health.
Even if a cumulative chronic low dose is equivalent to a dangerously high acute dose, since it accumulates over time, it is not as harmful. The body’s adaptive protection systems are effective in repairing radiation-induced damage from chronic low doses.
The next article in this series will describe both pros and cons of radiation exposure. Click here to read: Understanding Radiation Risks & Benefits.