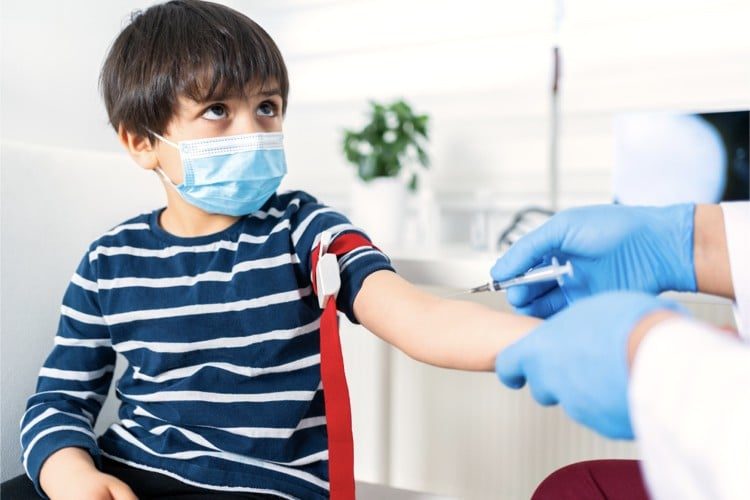
Pfizer and its partner BioNTech asked the U.S. government Thursday to allow use of its COVID shot in children aged 5 to 11. If the regulators find it “safe and effective” and the Center for Disease Control and Prevention (CDC) agrees, the inoculation of the young Americans could begin within a month.
According to the announcement made by the pharma giant on Twitter, “With new cases in children in the U.S. continuing to be at a high level, this submission is an important step in our ongoing effort against #COVID19.”
Even before Pfizer and BioNTech submitted the safety data to the Food and Drug Administration (FDA) for review, the latter scheduled a meeting of its Advisory Committee of Immunization Practices to discuss the issue on October 26. On Friday, though, the committee, which helps the CDC develop recommendations for doctors and the public about which vaccines should be used and how they should given, has reportedly rescheduled the discussion for November 2-3.
On September 20, Pfizer/BioNTech announced results from a Phase 2/3 trial in children 5 to 11 years of age “showing a favorable safety profile and robust neutralizing antibody.” The regimen used in children was the same as in adults: two doses given 21 days apart. Participants received a third of a dose given to grown-ups, and showed to generate an antibody responses comparable to those recorded in people 16 to 25 years of age immunized with the full dose, per the announcement.
It is also provided that the early phases of the trials enrolled some 4,500 children ages 6 months to 11 years of age in the United States, Finland, Poland, and Spain. The final phase, started in June, enrolled 2,268 participants who were 5 to 11 years-old.
Following the Pfizer’s announcement and the submission of the safety data to the agency, FDA commissioner Janet Woodcock promised the agency would review it “as quickly as we can.”
While the typical timeline for the vaccine development spans from 5 to 10 years, with phase III clinical trials taking some 2-4 years, Pfizer has spent 7 months to test its jab on children. The New American has previously reported that Pfizer started testing its jabs on healthy 6-month to 11-year old children just in the end of March 2021, which means that no mid- and long-term side effects of shots could possibly have been studied by now. Given the limited number of participants, it is also unlikely that the rare adverse reactions to the jabs could be recorded. Considering the already existing concerns in COVID jabs injuring and killing tens, if not hundreds of thousands of Americans, several months of jabs’ safety data in children does not look sufficient enough to recommend it to some 28 million children.
How justified the children’s inoculation with Pfizer jab would be, really? Do the benefits outweigh the risks?
According to the National Institute of Health (NIH), “Most children with SARS-CoV-2 infection will not require any specific therapy.” The institute implies that the healthy children are not generally at risk of getting COVID, let alone suffering severe complications from it, while noting that groups at risk include children who have a history of medical complexity (e.g., due to neurologic impairment, developmental delays, or genetic syndromes), obesity, chronic cardiopulmonary disease, or who are immunocompromised. The NIH also includes “nonwhite children and older teenagers” into that cohort.
All in all, children’s chances of dying of COVID are almost nonexistent thanks to the specifics of their immune system, which showed to be better equipped to eliminate SARS-CoV-2 than is adults’.
At the same time, studies indicate children are more likely to suffer from adverse reactions to the experimental COVID injections than they are to become seriously ill from COVID.
A safety report published last month by the Federal Institute for Vaccines and Biomedicines (Germany) found that the COVID jabs pose greater risk of health complications than catching COVID. The study showed that the number of reported cases of suspected adverse effects following COVID vaccination in children aged 12 to 17 has exceeded the total number of COVID-related hospitalizations for children within the same age group since the beginning of the pandemic.
A major study conducted at the University of California found that teenage boys are six times more likely to suffer from heart problems from the vaccine than be hospitalised from COVID.
Despite all the evidence that a) children are spared of risks of COVID and b) jabs may pose a significant threat to their health, the FDA has seen an enormous amount of pressure from the politicians and a part of the medical community to swiftly approve a COVID shot for young children.
In the end of August, more than 100 House lawmakers on both sides of the aisle wrote to the FDA asking for an update on its timeline for vaccines for children, citing the current “alarming” situation.
Around the same time, the American Academy of Pediatrics (AAP), considered the world’s largest and most influential professional association for pediatricians, and also the largest pediatric publisher, sent a letter to the FDA urging the agency to authorize the vaccine for children under 12 “as swiftly as possible.” To those who believe in authority of expertise and science, it would also be useful to know that the judgement of the seemingly respectable organization might be marred with a financial interest, since Pfizer is its major donor — a fact the AAP tried unsuccessfully to hide.
The top health officials, pushing for the vaccinations of the adolescents who are already eligible to receive a jab, have complaint of the high number of “cases” of Delta strain of COVID among children, even though the “case” usually means someone testing positive for COVID, and not necessarily falling ill or experiencing any symptoms at all.
The White House’s top medical advisor, Dr. Anthoiny Fauci, said earlier this month that “we have underestimated the impact [of COVID] on children,” and cited the high number of children being hospitalized “with COVID.”
Peter Marks, the director of the Center for Biologics Evaluation and Research (CBER) at the FDA, said earlier this week that the fact that 645 children died from a “preventable disease” such as COVID is “an embarrassment” for America.
“No parent should have to lose their child to a vaccine-preventable illness if we have a vaccine that can be deployed that is safe and effective. And we will only allow something to be authorized that we find to be safe and effective,” Marks added. Marks did not say how many young children and teenagers died of suicides, and drug overdoses, or how many were killed, abused or neglected during the past year, mostly because of the COVID-related policies imposed on millions of Americans, and whether he considered it an “embarrassment.”
In the meantime, it is expected that once the jab gets regulatory approval, the Biden administration and the “progressive” states would push to include 5-11-years-olds to the ever-expanding list of populations subjected to the mandatory vaccination.
California Governor Gavin Newsom announced on October 1 he would make the injections mandatory for all “eligible” students participating in in-person learning at both public and private schools.


