“The trouble with tritium is there is no trouble with tritium.” That’s the opening line in an article by James Conca that appeared in the October issue of Nuclear News. It addressed the issue of discharging Fukushima tritiated water to the Pacific Ocean, which is scheduled to begin in 2023.
My aim in this article is to focus on the biological aspects of tritium in the human body. We’ll find that tritium is nothing more than a “political” problem, generating lots of sound and fury from the media. Otherwise, it poses no threat to humans or the environment.
Nevertheless, there are a lot of people who are very angry with the decision to discharge tritiated water. As usual, a small contingent of them know it’s not a genuine health concern, but spread false information to advance an anti-nuclear agenda. There are many more who serve as useful idiots by uncritically repeating the disinformation. And there are the masses who become frightened because they willingly believe what they are told.
What Is Tritium?
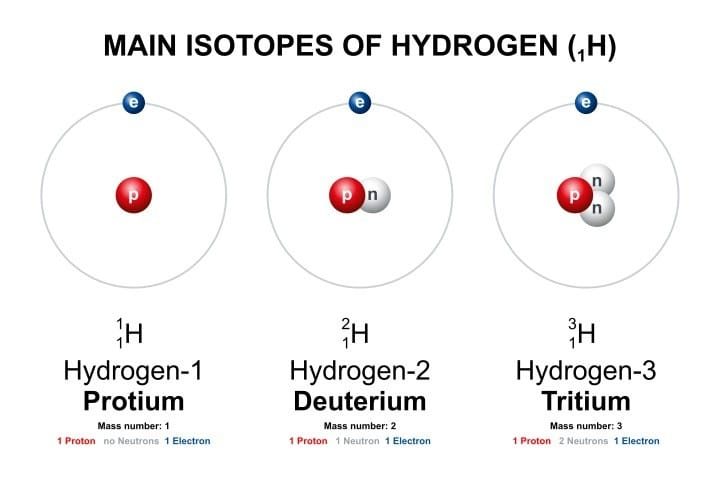
All that is necessary to combat such disinformation and dispel the ensuing irrational fear is a basic understanding of tritium. It is a radioactive isotope of hydrogen as shown in the graphic below.
The tritium atomic nucleus contains the proton of the normal hydrogen atom (protium) along with two neutrons. Most tritium is created in the Earth’s upper atmosphere when energetic cosmic neutrons interact with nitrogen atoms. It is also created in nuclear reactors where some tritium atoms can replace the protium atom in reactor cooling water (H2O) to produce tritiated water (HTO).
The contaminated water from the Fukushima nuclear power plant accident has been treated to reduce all radioactive substances to below regulatory discharge limits — except for tritium. That is because tritium cannot be removed from water; it is chemically indistinguishable from normal hydrogen (protium). In fact, according to the U.S. Environmental Protection Agency website, tritium is most commonly found in water “because radioactive tritium reacts with oxygen to form water.”
Biological Effects
Ostensibly, the health concern with tritium is from internal radiation exposure. Consider first the biological aspects of ingested tritium, for which there is no observed concentration in biological material.
With respect to its radioactivity, tritium has the weakest radioactivity of any radionuclide, with a beta particle maximum energy of 18.6 keV (“kiloelectronvolt,” or about 3 x 10-15 Joule). This low-energy beta particle has an extremely limited range in biological material of less than 10 micrometers (μm, or 10 x 10-6 meter). Note that the average diameter of a human cell is about 100 μm. Since roughly 60 percent of human body weight is water, tritium radioactivity in the human body is about 59 Becquerels (Bq) per liter. (One Bq is defined as one radioactive decay per second.)
If those units are confusing for the layman without any nuclear background, here’s a practical comparison. The radioactivity in whiskey is 44.4 Bq per liter, and in salad oil, it’s 181.5 Bq per liter. As Conca pointed out, tritium is so harmless that maximum regulatory limits in drinking water range from a low of 100 Bq/liter (European Union) to a high of 76,000 Bq/liter (Australia), as illustrated by the following bar chart.
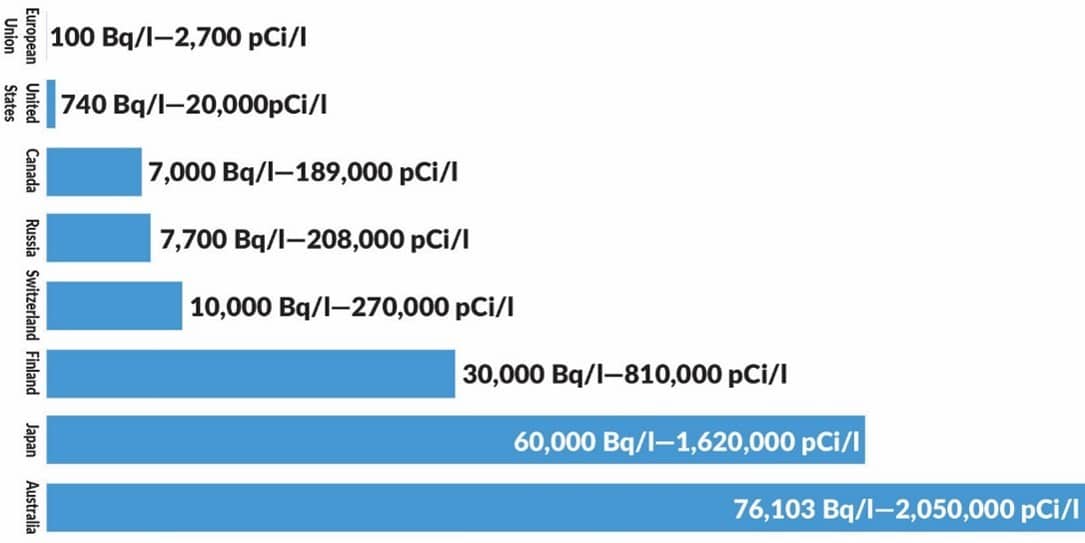
Note that the tritium radioactivity in the human body is well below the EU regulatory limit for drinking water. Incidentally, the other measurements listed in the graphic are equivalent conversions to picocuries per liter (pCi/l).
Tritium has a radiological half-life of 12.3 years, but its biological half-life is only about 10 days. Whereas the radiological half-life is the time taken for the amount of a radioactive substance to decrease to half of its initial value by radioactive decay, the biological half-life is the time taken for the amount of a particular element in the body to decrease to half of its initial value due to elimination by biological processes alone. The primary pathways for tritium excretion from the body are in urine (55 percent), through the skin (29 percent), and in exhaled air (12 percent).
Other Non-emergencies
Next, consider two radionuclides that present internal radiation exposures that aren’t health concerns — carbon-14 (C-14) and potassium-40 (K-40). C-14 is plentiful in the human body because we are carbon-based life forms. About 23 percent of human body weight is carbon, and there is one C-14 atom for every trillion carbon atoms.
The element potassium makes up about 0.18 percent of body weight and is found everywhere in the human environment. K-40 makes up only about 0.01 percent of naturally occurring potassium, but is the most common radioisotope in the human body.
Ninety percent or more of the C-14 and K-40 entering the human gastrointestinal tract is absorbed and enters the blood stream to be distributed throughout the body. Similar to tritium, C-14 and K-40 radioactively decay by beta particle emission. Unlike tritium decay, a gamma-ray is also emitted in the K-40 radioactive decay process.
C-14 has a radiological half-life of 5,730 years and emits a beta particle with an energy of 157 keV with a range in biological material of about 300 μm. The biological half-life of C-14 is about 40 days, with the primary excretion pathways being feces and exhalation.
K-40, with a radiological half-life of 1.26 x 109 years, emits a beta particle with an energy of 1,330 keV and a 1,461 keV gamma-ray. The average biological half-life is about 16 days and the primary excretion pathway is through urine. K-40 is consumed in much of the food we eat. The K-40 activity for some selected food items is shown in the following table (activity is given in picocuries per kilogram, where 1 pCi = 0.037 Bq).
| Food | 40K (β,γ) pCi/kg |
| Brazil nuts | 5,600 |
| Lima beans | 4,640 |
| Bananas | 3,520 |
| White potato | 3,400 |
| Carrot | 3,400 |
| Red meat | 3,000 |
| Low-sodium salt | 3,000 |
| Beer | 390 |
| Drinking water | – – – |
The biological aspects of tritium, C-14, and K-40 are summarized in the following table. By this information we can easily see that if C-14 and K-40 are not considered health hazards, there is no reason to be concerned about tritium as a health hazard.
| Radioactive Isotope | Radiological Half-life (years) | Biological Half-life | Radiation & Energy | Isotope Mass in Body (grams) | Activity within Body (Becquerels) |
| Potassium-40 | 1.26 x 109 | 16 days | β (1330 keV) γ (1461 keV) | 0.0165 | 4,340 |
| Carbon-14 | 5,730 | 40 days | β (157 keV) | 1.6 x 10-8 | 3,080 |
| Tritium (H-3) | 12.3 | 10 days | β (18.6 keV) | 2 x 10-14 | 7 |
Summary
Returning to the Fukushima tritiated water controversy, the concentration of tritium will be diluted with seawater to 1,500 Becquerels per liter (one-fortieth the concentration allowed by the Japanese drinking water safety standards) prior to ocean discharge via a one-kilometer-long undersea tunnel.
The final ocean concentration following a ten-year discharge will be about 2.3 x 10-6 Becquerel per liter. Therefore, the discharge plan will not pose any hazard to the environment, aquatic life, or humans.
Other articles in this series:
Dispelling Irrational Fear of Radiation
Understanding Radiation Risks & Benefits
The Dynamic World of Radioactive Decay
Latest Low-dose Radiation Research Program a Retread?
Radiation Dose and Cancer Risk




